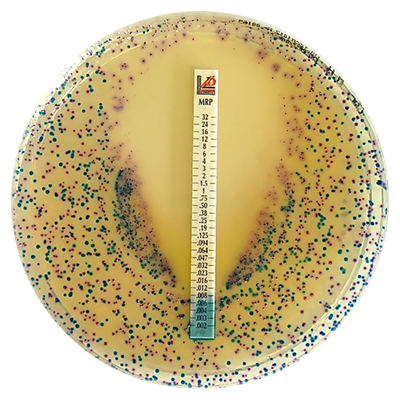
FDA Cleared MTS™
Availability in USA:
Ampicillin-sulbactam is the most recent FDA approved item in the MTS™ (MIC Test Strip) product range, following approvals of Vancomycin, Dalbavancin, Ceftolozane-tazobactam, Meropenem, Ceftazidime, Telavancin , Tedizolid , Delafloxacin , Clindamycin , Erythromycin, Linezolid, Meropenem-vaborbactam, Azithromycin, Ceftazidime-avibactam, Plazomicin, Penicillin G , Eravacycline, Omadacycline, Tetracycline, Levofloxacin, Ciprofloxacin, Gentamicin, Doxycycline, Imipenem-relebactm and Imipenem strips. The rest of the MTS™ range is available in the United States as research use only devices.
Availability in Europe and rest of the world:
The entire MTS™ product catalog is CE marked and fully available as IVD for clinical diagnostics purposes in Europe.
The MTS™ range is also compliant to MDSAP (Australia TGA, Brazil ANVISA, Health Canada, USA FDA, Japan MHLW/PMDA). It is registered at the competent Authority in many countries outside Europe as a clinical diagnostic device.
MTS™ Ampicillin-sulbactam |
MTS™ Imipenem |
MTS™ Imipenem-relebactam |
MTS™ Doxycycline |
MTS™ Gentamicin |
MTS™ Ciprofloxacin |
MTS™ Levofloxacin |
MTS™ Tetracycline |
MTS™ Omadacycline |
MTS™ Eravacycline |
MTS™ Penicillin G |
MTS™ Plazomicin |
MTS™ Delafloxacin |
MTS™ Azithromycin |
MTS™ Meropenem |
MTS™ Tedizolid |
MTS™ Erythromycin |
MTS™ Clindamycin |
MTS™ Meropenem-vaborbactam |
MTS™ Ceftazidime-avibactam |
MTS™ Linezolid |
MTS™ Telavancin |
MTS™ Ceftazidime |
MTS™ Ceftolozane-tazobactam |
MTS™ Dalbavancin |
MTS™ Vancomycin |